Crystal-liquid interfaces play an important role in many physical and chemical processes. Examples include catalysis, heterogeneous nucleation, and the overall shape of the crystal.
Ice nucleation at crystal surfaces is one topic that we have investigated extensively. In this area, we are currently interested in understanding how water behaves at K-feldspar and AgI surfaces, which are two of the best inorganice ice nucleating agents. Whereas our previous research has used classical intermolecular potentials, we are currently exploring the use of more accurate machine-learned interatomic potentials to describe these systems.
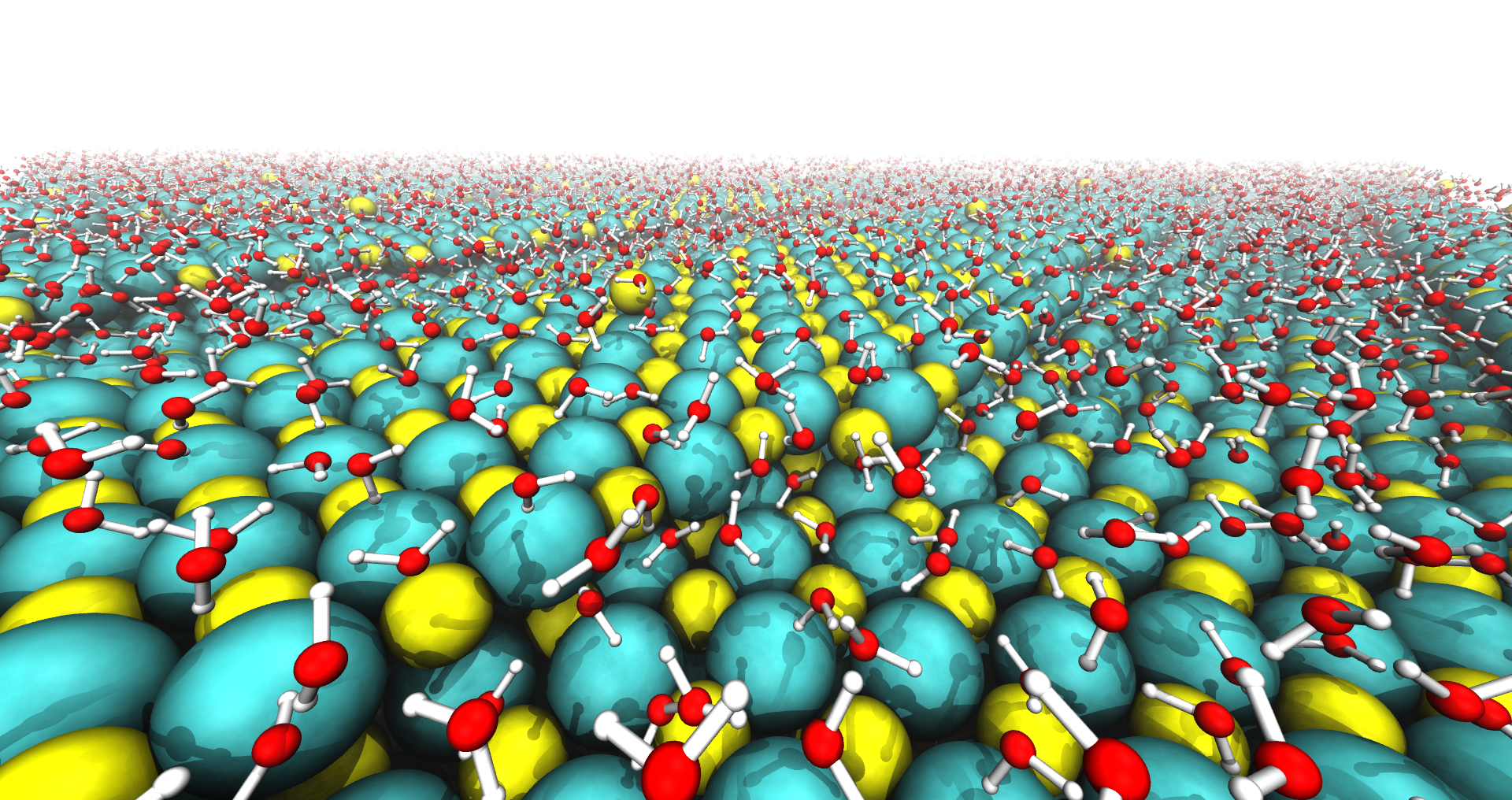
We also have a keen interest in polar crystal surfaces. We are interested in polar surfaces because they are catalytically active, can control the shapes of crystals (at both small and large length scales) and influence how particles in solution interact with each other. We also think their physical chemistry is fun! We have had to overcome many technical challenges when trying to simulate polar surfaces in contact with liquid solutions. We have found that standard simulation methods lead to qualitatively incorrect results whereby dissolved ions are repelled from charged crystal surfaces. This work on polar crystal surfaces is relevant to our studies on ice nucleation on AgI. But we are also interested in understanding how crystals exposing polar surfaces form from their melt, in a process known as “molten salt synthesis.”
Relevant Publications
- Stabilization of AgI's polar surfaces by the aqueous environment, and its implications for ice formation. T Sayer and SJ Cox, J. Chem. Phys. 21, 14546, 2019.
- A theory for the stabilization of polar crystal surfaces by a liquid environment. SJ Cox, J. Chem. Phys. 157 , 094701, 2022.
- Macroscopic surface charges from microscopic simulations. T Sayer, SJ Cox, J. Chem. Phys. 153 , 164709, 2020.